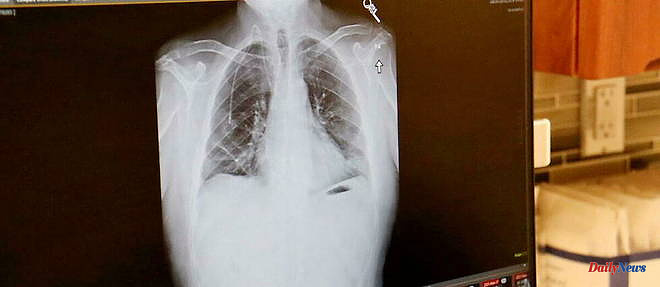
The hour of assessment has come for the high mass of world oncology. The American Society for Clinical Oncology meeting ends Monday, June 5 in Chicago. It brought together 43,000 experts from around the world to attend a 2023 edition marked by important announcements. Particularly in the area of lung cancer, which causes 1.8 million deaths worldwide, often related to smoking. In France, 46,000 new cases are detected each year, with a very large total of 33,000 deaths per year.
This clinical trial was conducted in 26 countries, on 682 patients who suffer from localized lung cancer, non-small cell, and without metastases. After chemotherapy, patients were either treated with the drug osimertinib (developed by the AstraZeneca laboratory, under the brand name Tagrisso), or received a placebo. 88% of patients treated with the molecule osimertinib were still alive five years after the start of treatment, compared to 78% of patients on placebo. Taking osimertinib resulted in a 51% reduction in the risk of death for treated patients compared to placebo.
“This post is making a big splash, and rightly so. Halving the risk of death at five years is a spectacular result,” adds Dr. Muriel Dahan, Director of Research and Development at Unicancer. Osimertinib is already authorized in dozens of countries and has been prescribed to some 700,000 people, according to a press release from AstraZeneca. Its approval in the United States in 2020 for the treatment of lung cancer was based on previous data. They had already shown an improvement in patient survival without cancer recurrence. But many doctors were waiting for data on overall patient survival. It's done.
The American Society for Clinical Oncology meeting also served to publicize advances in the field of ovarian cancer. “It is formidable because the five-year survival is less than 20%. So far, surgery has been the cornerstone of treatment," said Professor Jean-Marc Classe, head of the oncological surgery department at the West Cancer Institute (Nantes), also present in Chicago, on Monday.
The study he presented at Asco is titled CHIP, which stands for "chemo hyperthermia intraperitoneal". "HIPEC's efficacy hypothesis focuses on the specific treatment of the peritoneum, the membrane that lines the abdominal cavity with significant local concentrations of chemotherapy, associated with the effects of hyperthermia," he said Monday at Asco congress. It was carried out on 415 patients in cancer centers, in university hospitals, in France, but also in hospitals in Belgium and Spain. “The results are spectacular! The overall survival, without recurrence, of patients is postponed, on average, by one year compared to standard treatments", specifies Professor Jean-Marc Classe.
Among the avenues for the future, Professor Jean-Yves Blay, president of Unicancer, believes, after the various publications presented at this congress, that "conjugated antibodies are a major trend". Also nicknamed the "GPS" of cancer, in reference to the navigation system of cars, these antibodies make it possible to very precisely guide the molecule towards the targeted tumor. "Research over the next two or three years is going to be very important in this area, especially in the area of breast cancer," says Professor Blay. More than ever, the fight against cancer is no longer standardized. It is increasingly done à la carte, depending on each patient and the genetic identity of the tumours.