This will give them extra protection in the event of a rebound from the coronavirus.
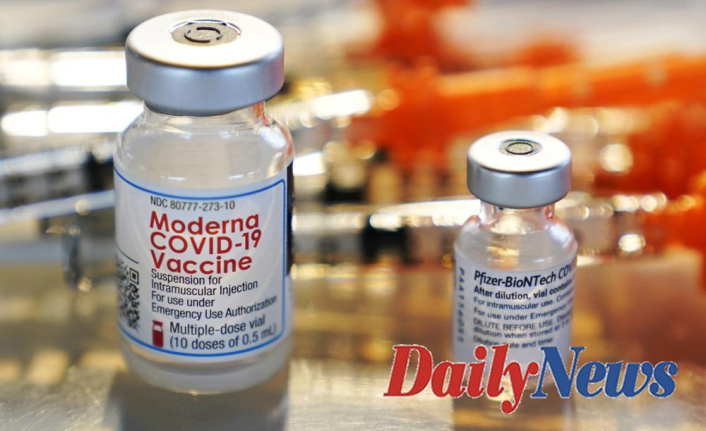
Tuesday's Food and Drug Administration approved an additional dose of the Pfizer Moderna or Moderna vaccine for this age group and certain younger people who have severely compromised immune systems.
Later, the Centers for Disease Control and Prevention recommended that an extra shot be considered. However, they did not urge anyone to rush out and order it immediately. This decision increases the number of Americans who can get an additional booster to millions.
Dr. Rochelle Walensky, CDC director, stated that it is especially important for seniors -- those over 65 -- and 50-somethings living with chronic diseases such as heart disease and diabetes to have another shot.
Walensky stated that "they are most likely to benefit from getting an additional booster dose at the time."
Evidence suggests that protection can decrease in high-risk groups. Dr. Peter Marks, FDA vaccine chief, said that another booster "will save lives".
While there has been much discussion about who should receive a fourth dose, less than half of Americans who are eligible have received one. The government encouraged them to do so. A booster and two shots are sufficient to protect against serious illness and death even in the winter surge of the super contagious omicron variety.
With limited evidence available to determine how much additional boosters could be of benefit, the move towards additional boosters is a sign of uncertainty. Although COVID-19 cases are down in the U.S., all vaccines against the newer variants of the virus are less effective than those used in earlier versions. Health officials are closely monitoring the omicron sibling that's causing alarming jumps in infection in other countries.
Pfizer asked for FDA approval for a fourth shot for those 65 years and over, while Moderna requested a dose for all adults to "provide flexibility" for government officials to determine who truly needs it.
Marks from FDA stated that regulators established the age limit at 50 to protect against chronic conditions like COVID-19.
The FDA has allowed a fourth dose of vaccines for immune-compromised children as young as 12. The FDA had previously allowed a fourth vaccine dose only for those with severely compromised immune systems. Tuesday's decision gives them another boost -- a fifth dose. Only the Pfizer vaccine is available for children under 12 years old; Moderna's can only be used for adults.
What about those who received Johnson & Johnson single-dose shots? They were already eligible for one booster. The CDC stated that 1.3 million people who received a second J&J shot may now be eligible for a third dose, either Moderna or Pfizer. The CDC states that an additional booster is not necessary for the over 4 million people who received Moderna or Pfizer as their 2nd shot. This is because they have a severely weakened immune system, or are 50 years old or older.
This is because a CDC study which tracked J&J booster recipients' initial choices concluded that a Moderna/Pfizer second dose was better than a J&J second dose.
Even if the new recommendations seem confusing, experts outside the field say that it is sensible to add protection for the most vulnerable.
E. John Wherry, an immunologist at the University of Pennsylvania, stated that there might be a reason for tanks to be topped up a bit for people with certain health conditions and older people. He wasn't involved with the government's decision.
He encourages his older relatives and friends to follow his advice. But Wherry, 50, who is well, vaccinated, and boosted, doesn't intend on getting another shot immediately. While I have strong protection against serious illness, I'm going to wait for it to become necessary.
Although protection against milder infections decreases over time, the immune systems builds multiple layers of defense. The one that protects against severe illness and death is the most durable.
Two doses of the U.S. omicron waves were almost 80% effective against death or needing a ventilator. A booster increased that protection to 94%, according to the CDC. Immune-compromised patients, who were the majority of those not receiving a third dose, had 74% less effectiveness than others.
U.S. officials sought out Israel to evaluate an additional booster. Israel opened a fourth dose for people over 60 during the omicron surge. In a review of 700,000.000 doses, the FDA found no safety issues.
Some preliminary data was posted online last week and suggested that there may be some benefit. Israeli researchers found that there were 92 deaths in the 328,000 who received the extra shot. This compares to the 232 deaths in the 234,000 who did not receive the fourth dose.
It's not clear how long an additional booster will last and when it should be used.
The 'when' part is the most difficult. We would prefer to time booster doses before surges but, as a vaccine expert at Johns Hopkins Bloomberg School of Public Health, Dr. William Moss said that it is not always possible.
A longer time interval between shots can help the immune system mount a stronger and more reactive defense.
Wherry stated that if you have boosters too close together it is not harmful -- it will not provide much benefit.
This booster expansion might not be the last. Next week, the government will hold an open meeting to discuss whether everyone needs a fourth dose of the original vaccine, or an updated shot.
Marks stated that even if Americans at higher risk are given a booster now, they might need another dose in fall if regulators make changes to the vaccine.
Studies in humans are ongoing to test omicron-targeted shots either alone or in combination. A booster that targets only omicron was found to be of no benefit by the National Institutes of Health.