If there had been no water on Earth, life would most likely never have appeared there. It must be said that its properties facilitate chemical reactions like no other liquid. The question of the origin of this precious fluid, particularly abundant on our planet, is therefore fundamental to better understand where we come from.
In an attempt to trace the Earth's water, scientists use its isotopic signature. Clearly, if a chemical element, such as hydrogen in water, corresponds to a single atom, there are however several varieties. These variants, called isotopes, have almost identical chemical properties, the same number of protons and electrons, but a different number of neutrons. In fact, there are therefore two types of water: ordinary water (H²O), made from hydrogen (H) devoid of neutrons, and heavy water (D²O), made from an isotope of hydrogen equipped with a neutron, deuterium (D), much rarer.
In nature, the two are mixed, so that by measuring the respective abundance of deuterium and hydrogen (D/H ratio) we obtain a kind of signature of the water studied. This is how scientists have, for example, noticed that meteorites, carbonaceous chondrites, true fossils from the formation of the solar system, incorporate hydrated minerals whose water has a D/H ratio – in other words a signature isotopic – very close to that of the water in our oceans.
But why is this D/H ratio particularly significant for tracing the origin of Earth's water? "To understand this, you have to know that all the hydrogen and all the deuterium that exists in the cosmos was formed at the beginning of the Universe and hasn't been made since. These are the first atoms from which all the other chemical elements were produced, by nuclear fusion, in the heart of successive generations of stars", explains the astronomer and chemist Cecilia Ceccarelli, researcher at the Institute of planetology and astrophysics of Grenoble. However, it is estimated that, at the beginning of the Universe, there was approximately 1 atom of deuterium for 100,000 atoms of hydrogen.
What about the D/H ratio of Earth's water? There are about 1 atom of deuterium for 10,000 atoms of hydrogen, ten times more: this is not insignificant! Earth's water has therefore undergone a transformation which means that it contains more heavy water than expected. In other words, one or more processes have enriched it in deuterium. But then, where and when?
ARCHIVEWater would have been present on the Earth since its formation
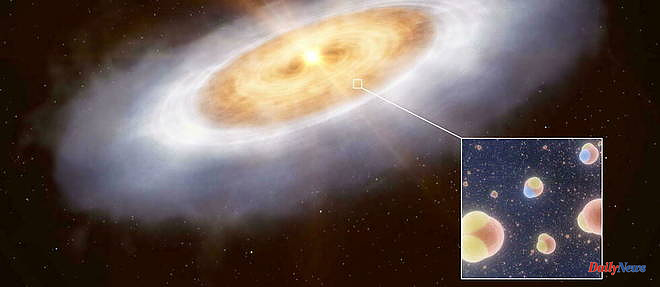
Indeed, astronomers have been able to detect gaseous water in a disk where planets are thought to form around the nascent star (or protostar) V883 Orionis, located about 1,300 years away. -Earth light. An object that is considered an analogue of the first moments of the formation of our own solar system. Not only were the researchers able to detect water around the protostar, but they were also able to "read" its isotopic signature.
As a result, its deuterium to hydrogen ratio is again surprisingly high, even higher than that of terrestrial water. It is therefore in the gas clouds where stars are formed that the water of planetary systems is enriched in deuterium. She who may have somewhere, like on Earth, made the bed of life.