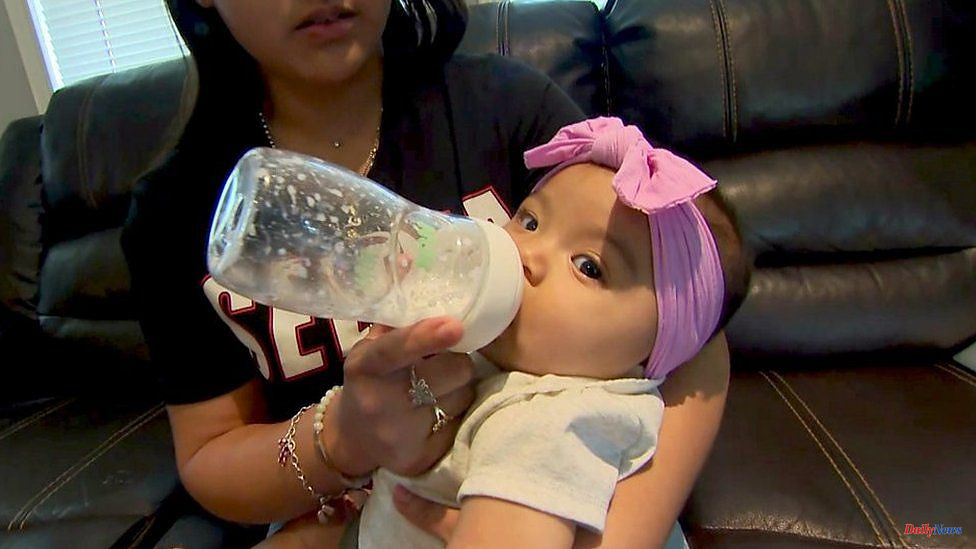Lauren Galvin, Mum, needed extra milk for her baby. After nursing, he was vomiting so much that he was not losing weight. He had to be admitted twice to the hospital.
However, the US was in severe shortage of infant formula so she couldn't buy any.
Lauren decided to go online and order a Dutch-made brand, which is not allowed to be imported to the United States.
The US authorities have warned repeatedly against purchasing milk that hasn’t been approved for export.
Customs officers confiscated $30,000 worth European formula last year. They claimed it didn't have the proper nutritional labelling and that they couldn't guarantee its safety. They also blocked $162,000 worth of formula at the border two years prior.
Lauren, a Missouri nurse, discussed the risks with her paediatrician, and other professionals, but she didn’t hesitate.
She says, "If this is healthy enough to be used by European babies, American babies will not be any different."
Lauren claims that the rules don’t make sense to him. She's not alone in having questions.
Now in its fourth month, the baby formula shortage has shaken trust in US food safety authorities. It revealed a combination of weak oversight and rigid rules that left the industry vulnerable to crises.
"I am angry," says New Jersey mum, Stephanie Esposito. Her nine-month-old son Dominic has severe allergies so she spends her days looking for formula. What are we supposed feed our babies?
"I don’t know how they can let it get this far."
The shortage began to manifest last year after Covid-related supply problems. After Similac formula maker Abbott Nutrition issued a major product recall in February, it reached full-blown crisis. One of the factories was shut down by authorities due to bacterial contamination. This resulted in a significant reduction in US production.
While few dispute the need to act on the conditions in the factory, the government's handling has been heavily criticised. This is especially after it was revealed by a whistleblower that authorities had known about sanitary issues for months before inspectors responded.
Analysts believe that the Food and Drug Administration (FDA), which supervises the formula industry made matters worse when it underestimated the capacity of Abbott's competitors to fill the gap in production. This was especially after panicked parents, which sent sales soaring.
By the age of three months, more than half of American infants have received at least some formula. Political pressure is mounting to find a solution.
Joe Biden, the US President, announced Operation Fly Formula last month. This allowed the FDA to temporarily relax its rules and approved the use of military jets for formula shipping from overseas.
More than twenty-six companies applied for formula imports into the US. Some of the brands that received waivers include Bubs Australia, Nestle's NAN, and Kendamil UK. These waivers are valid through November
Morvarid Rahmani, a professor of operations management at Georgia Tech’s Scheller College of Business, believes that if such moves had been made earlier, the crisis might have been avoided.
She says, "There is clearly not a global shortage." "All this could have been avoided if we took timely action."
However, there were doubts about FDA's ability to meet the shortage.
A wave of warnings was issued about the FDA's oversight of cosmetics, baby food, and sunscreen. Some parents began to use banned foreign baby milk products due to stricter regulations in certain overseas markets. This includes a ban on corn syrup (an ingredient that is common in US mix).
Mallory Whitmore (an Instagram influencer) is a certified infant-feeding technician, also known as "Formula Mom". She says that parents "no longer place a lot of trust" in FDA guidance.
"Especially right now we're witnessing this very rapid approval for foreign imports...which is very exciting but I think many parents are starting to wonder: Was the FDA really concerned about my infant's safety and health or about protecting American formula manufacturers?"
She points out that parents will not believe they are safe if the waiver for foreign formulas is removed. "The question is: "Are these foreign formulas going to remain or not?"
Analysts believe that increasing the supply of formula suppliers to the US is crucial for preventing another crisis.
However, the market is dominated by two major players: Abbott and Reckitt Benckiser’s Mead Johnson (which makes Enfamil) which together account for around 80%.
It's also difficult to break into.
Abbott and Reckitt Benckiser have many government contracts for formula supply to low-income families who are enrolled in the social support programmes. These programs account for approximately half of all US formula sales. New entrants will need to go through lengthy reviews of their formulas, as well as border taxes and strict labelling rules.
Despite falling birth rates and rising breastfeeding rates, US formula sales have been declining.
Thorben Nilewski is the managing director of Holle's US division, a Swiss formula maker. Although it has applied to distribute infant formula under the relaxed rules of Holle, he states that the regulatory hurdles for import are "very high".
"Holle has been following this field for a while but has never applied for admission to the university due to enormous cost and time costs," he said.
Laura Modi believes it's high time that the market is more open to competition. Bobbie, her own start-up, is a European-style formula that she launched after feeling frustrated by the lackluster options available to her as a mother.
She says, "When you look at why we're in this shortage it is inextricably linked to the fact only two players possess the majority of market share." "If one manufacturer is unable to compete, it should be possible to switch to another."
However, the FDA has maintained that current waivers for imports from overseas are temporary.
Last month, Robert Califf, the Commissioner of Washington, admitted to problems in food oversight. He said that the agency's other side needed a "shot in arm" and called for more funding and the power to monitor the supply chain. He has however defended the rules as they are consistent with safety standards.
Lauren believes that something must change. She says, "Do better." "Our children's lives are at stake."